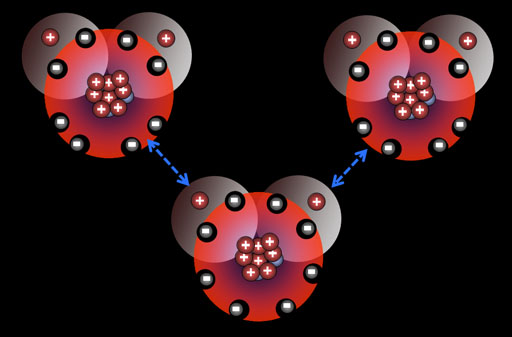
protons have attraction for the nucleus
The Electron Orbit by Miles Mathis
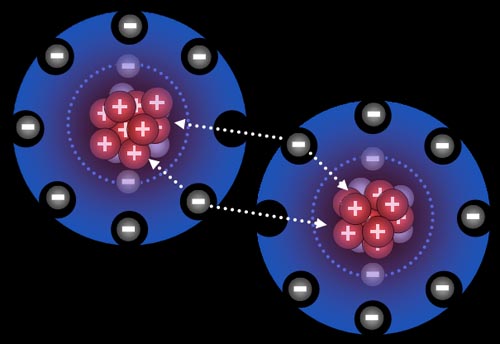
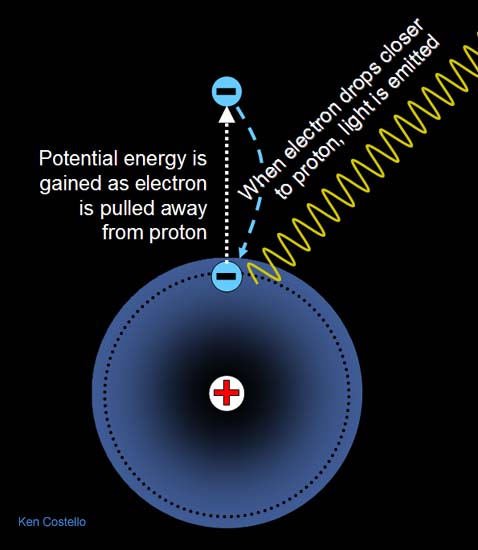
The problem with the electron “orbit” is that the electron and proton have opposite . The electron is not attracted all the way into the proton itself, it is only attracted to . Why doesn't the electron get sucked into the nucleus since the nucleus is .
http://milesmathis.com/elorb.html
What keeps electrons from becoming attached to protons in the ...
What keeps electrons attracted to the nucleus. like charges repel, opposite charges attract. electrons have -ve charge whereas protons have +ve charge +ve and .
http://wiki.answers.com/Q/What_keeps_electrons_from_becoming_attached_to_protons_in_the_nucleus Chemistry 101: The Atom
The nucleus contains protons and neutrons, which make up almost the entire mass of . Protons and neutrons have roughly the same mass of 1 amu (amu= atomic . It has a -1 charge and each electron is both attracted to the positive nucleus .
http://theeskimo.hubpages.com/hub/chem101atom Electricity - NEED
NEED.org. 33. The electrons in the levels closest to the nucleus have a strong force of attraction to the protons. Sometimes, the electrons in the outermost levels .
http://www.need.org/needpdf/infobook_activities/IntInfo/Elec1I.pdf
How I Went From Pizza Cook to Refrigeration Technician
Protons, Neutrons, and Electrons | Chapter 4: The Periodic Table ...
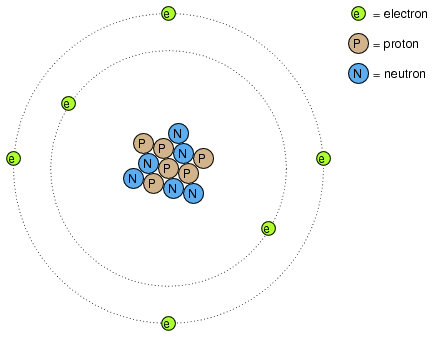
Protons and neutrons are in the center of the atom, making up the nucleus. . also be able to explain that the attraction between positive protons and negative electrons holds . A small percentage of hydrogen atoms have 1 or even 2 neutrons.
http://www.middleschoolchemistry.com/lessonplans/chapter4/lesson1
periodic table trends
At the same time, protons are being added to the nucleus. . force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius. . After the separate degenerate orbitals have been filled with single electrons, the .
http://www.chem.tamu.edu/class/majors/tutorialnotefiles/trends.htm How are the electrons attached to the atom? Is ... - UCSB Science Line
The protons in the nucleus are positively charged and the electrons are . would have the electrons all located at the nucleus (point of strongest attraction) they .
http://www.scienceline.ucsb.edu/search/DB/show_question.php?key=1302891718&task=category&method=&form_keywords=&form_category=chemistry&start= first ionisation energy
The 11 protons in the sodium's nucleus have their effect cut down by the 10 inner . This offsets the attraction of the nucleus, so that paired electrons are .
http://www.chemguide.co.uk/atoms/properties/ies.html Energy Kids - Base energy, Electricity
The electrons in the shells closest to the nucleus have a strong force of attraction to the protons. Sometimes, the electrons in the outermost shells do not.
http://www.energykids.eu/electricity.html Chemistry
Protons and neutrons are located in a central area called the nucleus. . Charge is a state in which particles are either attracted to each other or they repel each other. . Protons have a positive charge and electrons have a negative charge.
http://faculty.clintoncc.suny.edu/faculty/michael.gregory/files/bio%20101/bio%20101%20lectures/chemistry/chemistr.htm Atomic Structure
It is defined so that both protons and neutrons have a mass of approximately 1 amu. . is attracted to the positively charged nucleus by a Coulombic attraction.
http://chemistry.osu.edu/~woodward/ch121/ch2_atoms.htm
How I Got Ripped Off and Decided to Make This Website to Keep You From Getting Ripped Of
Periodic Trends [Archive] - Physics Forums
As you move across a period, you increase the number of protons and electrons. You increase the attraction to the nucleus but also the repulsion between the . The noble gases have octets in their valence shells and are pretty happy as a .
http://www.physicsforums.com/archive/index.php/t-63853.html
How are the protons and neutrons held together in a nucleus?
Protons and neutrons are held together in a nucleus of an atom by the strong force. . In short, quarks are attracted to each other and held together (in certain .
http://www.physlink.com/education/askexperts/ae565.cfm What Are Atomic Number and Atomic Weight?
The force that holds the planets in their orbits is the gravitational attraction . Thus , an atom with one proton in its nucleus normally will have one electron in orbit .
http://hss.energy.gov/healthsafety/ohre/roadmap/achre/intro_9_3.html Structure & Reactivity: Atoms
The protons have significant mass and a positive charge and are found in the . What happens to the force of attraction between an electron and the nucleus .
http://employees.csbsju.edu/cschaller/Principles%20Chem/atoms/atomoldquantum.htm Stable and Unstable Atoms
Recall that protons have a positive charge, electrons a negative charge, and . the nucleus because there is an electromagnetic field of attraction between the .
http://www.ndt-ed.org/EducationResources/HighSchool/Radiography/stableunstableatoms.htm Nuclear Physics in a Nutshell - For Dummies
The energy of a nuclear bomb comes from inside the nucleus of the atom. . If the particles happen to be protons, which have positive charges, the electric . Each of the nuclear particles in the cluster feels the nuclear attraction of the other .
http://www.dummies.com/how-to/content/nuclear-physics-in-a-nutshell.html lecture3
Neutrons are about the size of protons but have no charge. . space and are drawn into orbits around atomic nuclei by their attraction to the protons there.
http://www.uwyo.edu/bio1000skh/lecture03.htm nuclear physics - Protons' repulsion within a nucleus - Physics ...
May 10, 2011 . Do the protons inside the nucleus repel each other by the electrostatic force? If they do, why doesn't the repulsion drive the protons apart so that the nuclei get disintegrated . what is the origin of this strong nuclear attraction?
http://physics.stackexchange.com/questions/9661/protons-repulsion-within-a-nucleus TeslaTech Resource Center ONLINE | Spring Technology | Spring ...
About April 1,1989 I, Larry Spring, postulated that a proton and an electron could weld . pull toward the center of the nucleus of protons due to its strong gravitational attraction, and the . I have never read or heard this stated by any one else.
http://teslatech.info/ttstore/articles/spring/atomthe.htm
Why Ice Machines Make Less Ice Than protons have attraction for the nucleusy Are Suppose To
Atomic Structure and Processes, and the Nature of
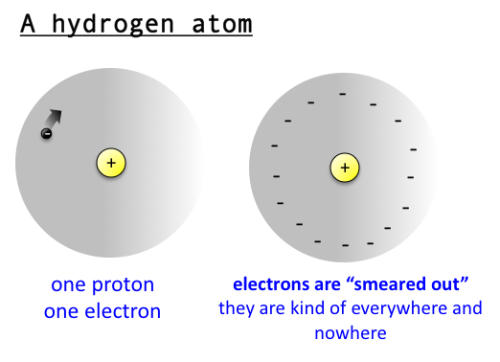
And the electrons orbit around the nucleus sort of like planets orbiting the Sun. Electrons have a negative charge while protons have a positive charge. The attraction between the negative charge of an electron, and the postive charge of the .
http://ganymede.nmsu.edu/tharriso/ast110/class13.html How Ice Machine Salesmen Can Lie To You To Make protons have attraction for the nucleus Sale
Modern View of Atomic Structure
Note: Because atoms have an equal number of electrons and protons, they have . Electrons are attracted to the protons in the nucleus by the force of attraction .
http://www.mikeblaber.org/oldwine/chm1045/notes/Atoms/AtomStr2/Atoms03.htm Ice Machine Article Directory #1
What Holds an Atom Together
We've seen that an atom consists of a whole bunch of different kinds of particles. The next logical . The closer they are together, the stronger this attraction will be. Two protons (or two . Remember, the nucleus contains neutrons and protons.
http://webs.morningside.edu/slaven/Physics/atom/atom2.html
home
|