lone pair of electrons
Lone pair - Wikipedia, the free encyclopedia
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone .
http://en.wikipedia.org/wiki/Lone_pair
Lewis structure - Wikipedia, the free encyclopedia
They are similar to electron dot diagrams in that the valence electrons in lone pairs are represented as dots, but they also contain lines to represent shared pairs .
http://en.wikipedia.org/wiki/Lewis_structure Why do lone pair electrons repel each other more strongly ?
Why do lone pair electrons repel each other more strongly ? Chemistry discussion.
http://www.physicsforums.com/showthread.php?t=360606 Molecular Geometry
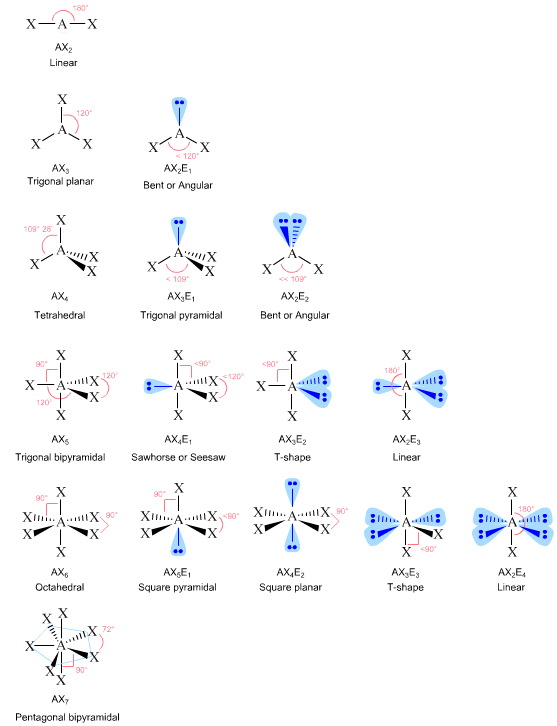
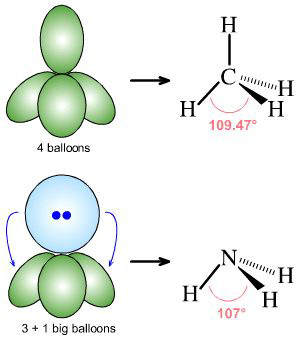
We will use a model called the Valence Shell Electron-Pair Repulsion (VSEPR) model that is based on the repulsive . of lone pair electrons on 'central' atom .
http://intro.chem.okstate.edu/1314f00/lecture/chapter10/vsepr.html
How I Went From Pizza Cook to Refrigeration Technician
How do lone pairs of electrons affect the molecular geometry of a ...
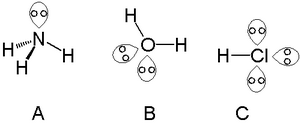
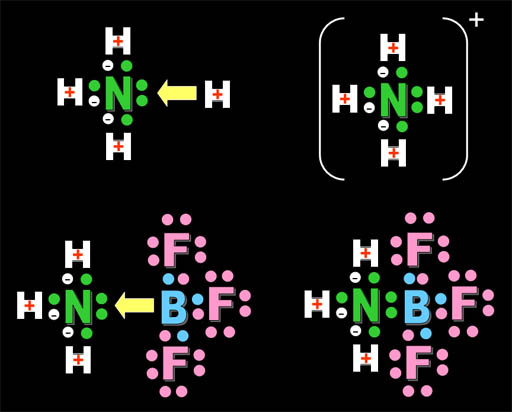
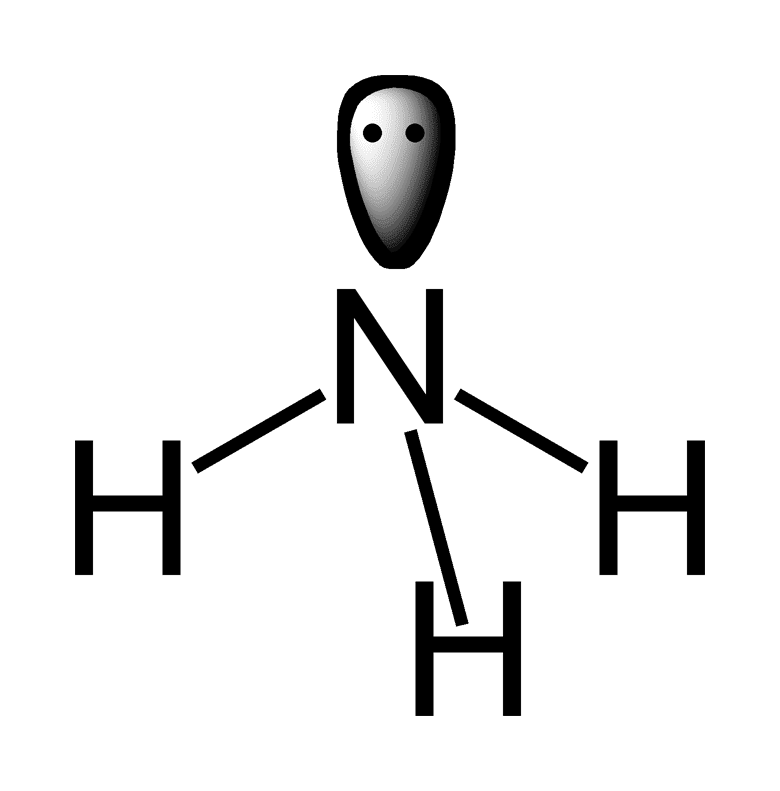
Does the ammonia molecule have a lone pair of electrons? Yes. 5 Valence electrons, 3 of which are shared with hydrogens. The remaining two are the lone pair .
http://wiki.answers.com/Q/How_do_lone_pairs_of_electrons_affect_the_molecular_geometry_of_a_molecule
Valence Shell Electron Pair Repulsion Theory
The Valence Shell Electron Pair Repulsion Theory is a way of predicting the shape of a molecule based on the number of bonding and lone pairs of electrons in .
http://www.everyscience.com/Chemistry/Inorganic/Molecular_Structure/b.1026.php lone pair - definition of lone pair by the Free Online Dictionary ...
The two electrons left over in the form of a lone pair, which makes this species highly reactive to attack CO2. New method converts CO2 into methanol under very .
http://www.thefreedictionary.com/lone+pair Hückel's Rule - ChemWiki
Mar 13, 2012 . The oxygen has at least 1 lone electron pair and is attached to an sp2 hybridized atom, so it . Notice how oxygen has 2 lone pairs of electrons.
http://chemwiki.ucdavis.edu/Organic_Chemistry/Aromatics/H%C3%BCckel's_Rule Modifications to basic shapes: considering the effect of lone pairs
Di-bromodimethylselenium (CSD refcode RIZMIW) has 5 electron pairs (4 bonding pairs, and 1 lone pair), the parent shape is therefore trigonal- bipyramidal.
http://www.ccdc.cam.ac.uk/support/documentation/csd/teaching_egs/teaching_examples.3.27.html Chemistry Tutorial: Aromaticity
of the overall structure and electron delocalization of the molecule, the molecule is aromatic. ? What do lone pairs have to do with aromaticity? o Lone pairs can .
http://www.chem.ucla.edu/harding/tutorials/aromaticity.pdf • Trigonal planar electron-group arrangement with one lone pair ...
Trigonal planar electron-group arrangement with one lone pair. ?Two atoms attached to the central atom + one lone pair (AX2E) ? Bent shape. ?The lone pair .
http://g.web.umkc.edu/gounevt/Weblec211Silb/L36(10.3-10.4).pdf
How I Got Ripped Off and Decided to Make This Website to Keep You From Getting Ripped Of
ELECTRON PAIR REPULSION THEORY- HYBRID ORBITAL MODEL
Following are the main points of electron pair repulsion theory: There are two types of electron pairs surrounding the central atom. Bond pair. Lone pair.
http://www.citycollegiate.com/hybridization3.htm
Phys. Rev. B 85, 085207 (2012): Role of lone pair electrons in ...
Feb 16, 2012 . Rev. B 85, 085207 (2012) [6 pages]. Role of lone pair electrons in determining the optoelectronic properties of BiCuOSe. Abstract. References .
http://link.aps.org/doi/10.1103/PhysRevB.85.085207 ROCO Resonance: Delocalized
According to this theory, localized electrons exhibit normal behavior. A localized lone pair remains close to one atom. A localized bond pair travels between two .
http://academic.reed.edu/chemistry/roco/resonance/delocalized.html MOLECULAR MODELS (Unshared Electron Pair)
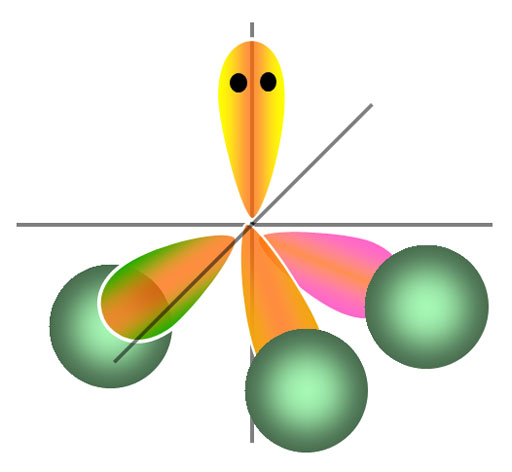
An unshared electron pair, also known as a nonbonding pair of electrons or as a lone pair of electrons, is two electrons in the same orbital in the outer shell of an .
http://www.eou.edu/chemweb/molmodel/mmp4b.html ELECTRON DELOCALIZATION AND RESONANCE
The following example illustrates how a lone pair of electrons from carbon can be moved to make a new pi bond to an adjacent carbon, and how the pi electrons .
http://www.utdallas.edu/~scortes/ochem/OChem1_Lecture/Class_Materials/06_electr_delocal_res.pdf Electron Pushing in Organic Chemistry
This handout deals with electron pushing arrows: the movement of a pair of electrons from an electron rich site (a lone pair of electrons or a bond) to an electron .
http://www.chem.wisc.edu/areas/reich/handouts/elecpush/epush-1.htm A Sure-Fire Way to Draw Lewis Structures!
6) Find the number of lone pair (nonbonding) electrons by subtracting the bonding electrons (#3 above) from the valence electrons (#1 above). Arrange these .
http://misterguch.brinkster.net/lewisstructures.html Stereochemical activity of lone pairs of electrons and ...
The coordination geometries are rich and varied due to the stereochemical influence exerted by up to two lone pairs of electrons and the penchant of tellurium to .
http://www.sciencedirect.com/science/article/pii/S0010854509002239 The use of curly arrows to show electron movements in reaction ...
The arrow tail is where the electron pair starts from. That's always fairly obvious, but you must show the electron pair either as a bond or, if it is a lone pair, as a .
http://www.chemguide.co.uk/basicorg/conventions/curlies.html
Why Ice Machines Make Less Ice Than lone pair of electronsy Are Suppose To
Molecular Geometry - Introduction to Molecular Geometry
There are two electron pairs around the central atom in a molecule with linear molecular geometry, 2 bonding electron pairs and 0 lone pairs. The ideal bond .
http://chemistry.about.com/od/atomicmolecularstructure/a/moleculargeometry.htm How Ice Machine Salesmen Can Lie To You To Make lone pair of electrons Sale
Electron pair geometry
The geometry is determined by minimizing the repulsions between electron pairs in the bonds between atoms and/or lone pairs of electrons as postulated by .
http://www.elmhurst.edu/~chm/vchembook/207epgeom.html Ice Machine Article Directory #1
Electron pair - Wikipedia, the free encyclopedia
a chemical bond between two atoms; as a lone pair. fill the core levels of an atom . Because the spins are paired, the magnetic moment of the electrons cancels .
http://en.wikipedia.org/wiki/Electron_pair
home
|